Materials & Methods
Obtaining A Representative Sample
- Stir and obtain 3 different samples of a single brand of orange juice
-Then mix the 3 samples together and stir to make it homogeneous
Sample Preparation:
- Spike 0.5 mL of Orange Juice with Triphenyl Phosphate (as an Internal Standard) at 50 ppb.
- Dilute 3:1 with HPLC Grade Water & vortex
- Centrifuge 15-20 min at 6000 rpm to remove the sediments
- Collect supernatant which the carbendazim dissolves in
- Run HPLC/MC analysis
Internal Standard Calibration Method:
- Prepare 5 known standards of Carbendazim of different concentrations, with Triphenyl Phosphate as the internal standard.
- Inject each standard and obtain the detector response at each concentration
- Draw a calibration graph between normalized peak areas and concentration of standard solutions to obtain the Calibration Graph
Sample Quantification:
- Inject prepared samples into HPLC and obtain the detector response
- Calculate the concentration of carbendazim from the Calibration Graph
----------------------------
Triphenyl phosphate is used as the internal standard because it is available in high purity (>99% from Sigma-Aldrich), and chemically similar and does not react with Carbendazim and the other fungicides of interest in the study. In addition, it does not cause any chemical or matrix interference in the chromatographic process, and its physico-chemical properties are similar to carbendazim.
----------------------------
Results & Discussion
Here are the MRM extracted ion chromatographs for Carbendazim (spiked at 2 ppb) & Triphenyl phosphate (spiked at 50 ppb) in orange juice extract from the study.
Carbendazim was spiked in the orange juice extract to simulate a scenario of orange juice contaminated with the fungicide carbendazim.
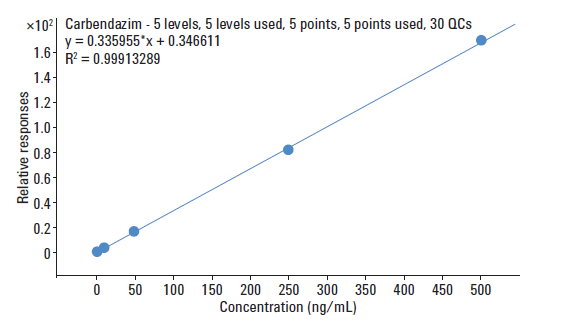
This is the calibration graph for the concentration of carbendazim in orange juice extract, from 2.0 - 500 ppb concentration levels.
References:
n.d. 2012. LC/MS/MS of Fungicides and Metabolites in Orange Juice with Agilent Bond Elut Plexa and Poroshell 120. [ONLINE] Available at: http://www.chem.agilent.com/Library/applications/5991-0051EN.pdf. [Accessed 27 January 2013].
n.d. 2012. The Determination of Benomyl and Carbendazim in Municipal and Industrial Wastewater. [ONLINE] Available at: http://water.epa.gov/scitech/methods/cwa/bioindicators/upload/2007_11_06_methods_method_631.pdf. [Accessed 17 January 2013].
n.d. 2012. LC/MS/MS of Fungicides and Metabolites in Orange Juice with Agilent Bond Elut Plexa and Poroshell 120. [ONLINE] Available at: http://www.chem.agilent.com/Library/applications/5991-0051EN.pdf. [Accessed 27 January 2013].
n.d. 2012. The Determination of Benomyl and Carbendazim in Municipal and Industrial Wastewater. [ONLINE] Available at: http://water.epa.gov/scitech/methods/cwa/bioindicators/upload/2007_11_06_methods_method_631.pdf. [Accessed 17 January 2013].